Carbon dots with tunable concentrations of trapped anti-oxidant as an efficient metal-free catalyst for electrochemical water oxidation†
Abstract
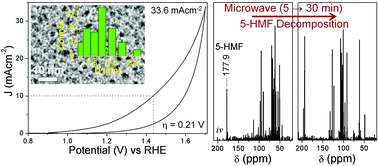
The challenging water oxidation reaction to generate molecular oxygen requires low-cost efficient catalysts for its application in renewable energy technologies. Carbon dot (C-dot) catalysts synthesized by microwave irradiation can trap an anti-oxidant, 5-hydroxymethyl-2-furaldehyde (5-HMF) inside the carbon framework. The C-dot with the highest concentration of 5-HMF acts as a stable metal-free oxygen evolution reaction (OER) catalyst which operates at a decently low 0.21(±0.03) V overpotential and can generate current density up to 33.6(±2.3) mA cm−2. With increased microwave reaction time, the concentration of 5-HMF inside the C-dots decreases at the cost of different furan derivatives which decreases the OER activity. The 5-HMF molecules in close vicinity to the catalytically active sites containing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups can extract the ˙OH/˙OOH radicals and can increase the in situ H2O concentration to facilitate the forward reaction of O2 evolution. During continuous electrolysis beyond 10 min, 5-HMF gets converted to 2,5-diformylfuran entities, which increases the catalytically active sites and thereby maintains the OER activity of the C-dots for at least 4 h. The ability of microwave irradiated sucrose derived C-dots to electro-oxidize water is generalized with C-dots and graphene dots (G-dots) prepared from different precursors.
O groups can extract the ˙OH/˙OOH radicals and can increase the in situ H2O concentration to facilitate the forward reaction of O2 evolution. During continuous electrolysis beyond 10 min, 5-HMF gets converted to 2,5-diformylfuran entities, which increases the catalytically active sites and thereby maintains the OER activity of the C-dots for at least 4 h. The ability of microwave irradiated sucrose derived C-dots to electro-oxidize water is generalized with C-dots and graphene dots (G-dots) prepared from different precursors.

 Please wait while we load your content...
Please wait while we load your content...