Ultrahigh-rate-capability of a layered double hydroxide supercapacitor based on a self-generated electrolyte reservoir†
Abstract
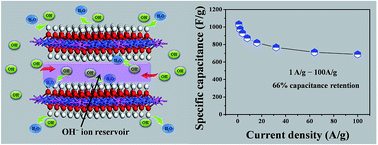
A hierarchical CoAl–OH layered double hydroxide (H-OH-LDH) electrode was prepared via a continuous calcination–rehydration treatment of a plate-like CoAl–CO3 layered double hydroxide (P-CO3-LDH) array on a nickel foil substrate. The H-OH-LDH electrode shows a well-defined hierarchical structure with a greatly increased accessible interlaminar surface area, leading to improved electrochemical energy storage ability. Most significantly, the interlayer space of H-OH-LDH acts as an electrolyte micro-reservoir to store OH− ions, which dramatically decreases the diffusion resistance of OH− to the inner surface of LDH lamella, and consequently results in an ultrahigh-rate-capability (capacitance reservation of 66% when the current density increases from 1 to 100 A g−1). The remarkable rate capability is superior to that of ever-reported transition metal oxide/hydroxide-based electrodes. In addition, an all-solid-state hybrid capacitor device was fabricated based on this H-OH-LDH electrode, exhibiting outstanding energy and power output (35.5 W h kg−1 at 27.3 kW kg−1) as well as excellent cycling stability. Therefore, this work demonstrates a new approach for the design and fabrication of LDH-based materials with self-generated electrolyte reservoirs, which have promising potential application in energy storage/conversion systems.

 Please wait while we load your content...
Please wait while we load your content...