Ultra-durable two-electrode Zn–air secondary batteries based on bifunctional titania nanocatalysts: a Co2+ dopant boosts the electrochemical activity†
Abstract
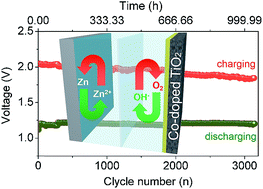
High energy density, low cost and ultra-stable bifunctional electrocatalysts that act simultaneously for the oxygen reduction reaction (ORR) and the oxygen evolution reaction (OER) are important for commercializing the rechargeable Zn–air batteries. Co-doped TiO2 nanoparticles as an ultra-stable and cheap electrocatalyst exhibited excellent activity for both ORR and OER in alkaline media. A real air cathode made of the Co-doped TiO2 electrocatalyst further offered superior high energy density (778 mA h gZn−1 and 938.5 W h kgZn−1 at 5 mA cm−2, and 785.9 mA h gZn−1 and 911.3 W h kgZn−1 at 20 mA cm−2) and ultra-high stability (37 cycles for 750 h of operation at 20 mA cm−2 and 3150 cycles for 1050 h of operation at 5 mA cm−2) in two-electrode Zn–air batteries.

 Please wait while we load your content...
Please wait while we load your content...