Direct photocatalytic hydrogen evolution from water splitting using nanostructures of hydrate organic small molecule as photocatalysts†
Abstract
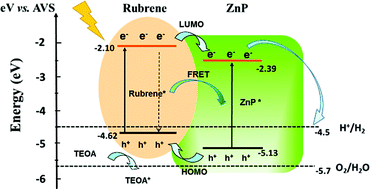
Organic small molecules with a suitable energy level have usually been considered as photosensitizers rather than catalysts for photocatalytic hydrogen evolution (PHE). Herein, we achieved direct PHE using hydrate zinc tetraphenylporphyrin (ZnP, ZnTPP·H2O) nanostructures synthesized by a liquid-phase chemical reaction as photocatalysts. The shape-dependent photocatalysis revealed that the ZnP nanosheets (ZnP-NS) exhibit higher PHE activity (∼0.16 mmol g−1 h−1) than the ZnP octahedron nanoparticles (ZnP-NPs) (∼0.06 mmol g−1 h−1). After in situ construction of the rubrene/ZnP-NS heterostructure, more efficient PHE of this pure organic nanostructure was obtained due to the occurrence of photoinduced electron transfer and Förster resonance energy transfer (FRET). The optimal PHE rate is ∼0.56 mmol g−1 h−1. Furthermore, with the addition of 3.0 mM methyl viologen (MV) and 3.8 wt% platinum, a PHE rate of ∼9.3 mmol g−1 h−1 can be achieved at pH = 7. This study offers a new route to design organic small molecules as photocatalysts.

 Please wait while we load your content...
Please wait while we load your content...