An electron injection promoted highly efficient electrocatalyst of FeNi3@GR@Fe-NiOOH for oxygen evolution and rechargeable metal–air batteries†
Abstract
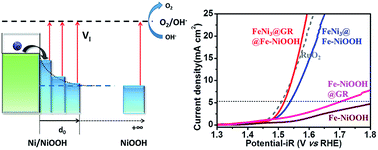
Efficient catalysts for oxygen evolution reactions (OERs) are a key renewable energy technology for fuel cells, metal–air batteries and water splitting, but few non-precious oxygen electrode catalysts with high activity have been discovered. Here, we propose a general strategy based on electron injection to manipulate the work function of electrocatalysts to obtain an extraordinary performance beyond precious catalysts. Based on the density functional theory calculation, the NiOOH/Ni hybrid reveals the smallest overpotential compared to NiOOH. A novel hybrid catalyst is designed to grow Fe-doped NiOOH on graphene-encapsulated FeNi3 nanodots (FeNi3@GR@Fe-NiOOH). Accordingly, the catalyst exhibits excellent OER activity and superior durability, affording a low onset potential of 1.45 V vs. reversible hydrogen electrode (RHE) and a stable current density of 11.0 mA cm−2 at 1.6 V (vs. RHE) for over 12 h. The achieved turnover frequency of 1.16 s−1 at an overpotential of 300 mV is the best performance among the reported similar catalysts, and even better than that of the state-of-the-art noble-metal catalysts (RuO2 and IrO2). The high electrocatalytic efficiency and robust durability are essential conditions for a superior air electrode material for Zn–air batteries. Our catalyst cycled stably for 360 cycles at 1 mA cm−2 in 20 h with no obvious attenuation over 100 cycles for 100 h.

 Please wait while we load your content...
Please wait while we load your content...