A clear coat from a water soluble precursor: a bioinspired paint concept†
Abstract
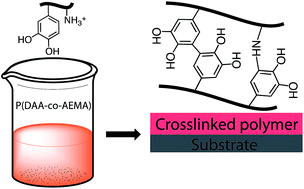
Traditional paints consist of hydrophobic polymers dissolved in hydrocarbons; they are appreciated for their rheological properties and the smooth and glossy films they form upon drying and crosslinking, but are now largely banned because of the hazards associated with the solvents. In terms of health, water borne paints based on colloidal resin dispersions are an improvement but these systems lack the rheological and film forming properties of traditional paints. We present here a bio-inspired alternative that combines the best of both worlds: a water soluble polymer that can crosslink by mild oxidation to a fully water resistant and adhesive coating. Using free radical polymerization, we copolymerized two water soluble monomers, namely borax-protected dopamine acrylamide (DAA-p) and 2-aminoethylmethacrylamide (AEMA) in various proportions. We determined the reactivity ratio of these monomers using an in situ1H NMR monitoring method and found values of 0.0 and 0.46 for DAA-p and AEMA, respectively. From this we conclude that in the polymers DAA-p pairs do not occur, while DAA–AEMA diads are relatively frequent. After removing the protective borax by hydrolysis, we obtain polymers which are soluble in water at low pH (pH 2) but which undergo rapid oxidative crosslinking when the catechol groups of DAA react with amines from AEMA. This leads to a water resistant, mildly hydrophobic film with a water/air contact angle of about 40 degrees which adheres well to glass substrates.
- This article is part of the themed collection: Emerging Investigators 2016: Novel design strategies for new functional materials

 Please wait while we load your content...
Please wait while we load your content...