Increased activity in hydrogen evolution electrocatalysis for partial anionic substitution in cobalt oxysulfide nanoparticles†
Abstract
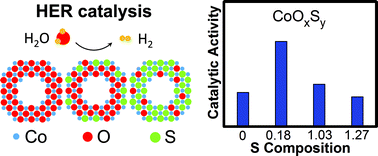
The functionality of an electrocatalyst depends sensitively on the surface chemistry. In the case of transition-metal compounds, both the transition metal cation and the anion must be controlled to maximize the electrocatalytic activity. This realization has driven many efforts devoted to engineering the cation chemistry, producing many state-of-the-art electrocatalysts. Motivated by the critical role the cation plays in electrocatalysis, we seek to understand whether a similar effect can be achieved with the anion. Herein, we present a study on the effect of the anion substitution on the hydrogen evolution reaction (HER) electrocatalysis on cobalt oxysulfide nanoparticles. To control the sulfur substitution, we use ammonium sulfide to introduce sulfur to the cobalt oxide nanoparticles at low temperature without inducing secondary phase formation. We find that a lightly doped oxysulfide catalyst, which has the composition CoOxS0.18, exhibits a metastable, distorted S-substituted CoO phase and is 2–3 times more active for the HER than either end-member of the substitution series. Our first-principles calculations attribute the HER enhancement to the stronger surface H adsorption which is maximally favorable at a relatively low doping level. Our work provides a protocol for synthesizing metastable mixed-anion materials and reveals the critical role of the anion on the surface physiochemical properties and the HER electrocatalysis.
- This article is part of the themed collection: Water splitting and photocatalysis


 Please wait while we load your content...
Please wait while we load your content...