Visible-light-driven photocatalytic bacterial inactivation and the mechanism of zinc oxysulfide under LED light irradiation†
Abstract
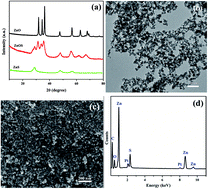
Zinc oxysulfide (ZnO0.6S0.4) nanoparticles, prepared via a coprecipitation–calcination method, were used as an effective visible-light-driven (VLD) photocatalyst for the inactivation of a typical Gram-negative bacterium, Escherichia coli K-12 for the first time. An energy-saving white light emitting diode (LED) lamp was employed as the visible light (VL) source. Compared to the only UV-responsive pure ZnO and ZnS, the light active region of ZnO0.6S0.4 was expanded as far as 550 nm in the VL region. Significantly, the obtained ZnO0.6S0.4 nanoparticles showed considerable VLD photocatalytic bacterial inactivation activity under white LED irradiation. The mechanism of inactivation was investigated in-depth. Photogenerated holes (h+) and hydrogen peroxide (H2O2) were predominantly responsible for the bacterial inactivation. Moreover, H2O2 was evidenced to be derived only from electrons in the conduction band of ZnO0.6S0.4 in the present photocatalytic system. The integrated damage from the direct oxidation effect of the h+ and continuous accumulation of H2O2 resulted in a high bacterial inactivation efficiency of ZnO0.6S0.4 nanoparticles under visible white LED lamp irradiation. The destruction process of bacterial cells by the ZnO0.6S0.4 photocatalyst was also monitored. This was shown to begin with an attack of the cell membrane and then end in the release of intracellular components.

 Please wait while we load your content...
Please wait while we load your content...