Sol–gel copper chromium delafossite thin films as stable oxide photocathodes for water splitting†
Abstract
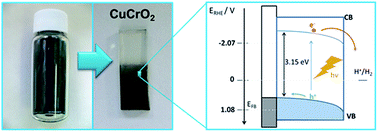
Significant effort is being devoted to the study of photoactive electrode materials for artificial photosynthesis devices. In this context, photocathodes promoting water reduction, based on earth-abundant elements and possessing stability under illumination, should be developed. Here, the photoelectrochemical behavior of CuCrO2 sol–gel thin film electrodes prepared on conducting glass is presented. The material, whose direct band gap is 3.15 eV, apparently presents a remarkable stability in both alkaline and acidic media. In 0.1 M HClO4 the material is significantly photoactive, with IPCE values at 350 nm and 0.36 V vs. RHE of over 6% for proton reduction and 23% for oxygen reduction. This response was obtained in the absence of charge extraction layers or co-catalysts, suggesting substantial room for optimization. The photocurrent onset potential is equal to 1.06 V vs. RHE in both alkaline and acidic media, which guarantees the combination of the material with different photoanodes such as Fe2O3 or WO3, potentially yielding bias-free water splitting devices.

 Please wait while we load your content...
Please wait while we load your content...