Substituent effect of Ru(ii)-based sensitizers bearing a terpyridine anchor and a pyridyl azolate ancillary for dye sensitized solar cells†
Abstract
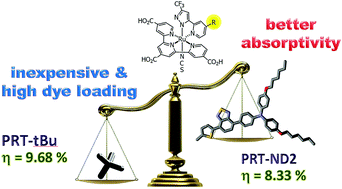
In this study we explore the substituent effects of a class of Ru(II)-based sensitizers bearing a 4,4′,4′′-tricarboxy-2,2′:6′,2′′-terpyridine anchor, a functional pyridinyl azolate and a single thiocyanate ligand. Three sensitizers, i.e.PRT-tBu, ND-1 and ND-2, with t-butyl, 5-[4-[bis(4-hexyloxyphenyl)amino]phenyl]-2-thienyl and 5-[7-[4-[bis(4-hexyloxyphenyl)amino]phenyl]-4-(2,1,3-benzothiadiazolyl)]-2-thienyl substituents are designed and synthesized. Their photophysical and redox properties are probed using UV/vis absorption and cyclic voltammetry studies. The DSC cell performances (JSC, VOC and PCE) were also examined and analyzed in order to elucidate the structure–property relationships. It is notable that all sensitizers showed superior spectral responses in the region up to 830 nm, among which the PRT-tBu and ND-1 showed prolonged electron lifetimes, suppressed electron recombination and higher power conversion efficiencies (PCE) compared to the third sensitizer PRT-ND2 with a benzothiadiazolyl linker at the ancillary chelate. In particular, the optimized DSC device using the PRT-tBu sensitizer gives a JSC of 16.29 mA cm−2, a VOC of 787 mV and a fill factor (FF) of 0.75, corresponding to an overall PCE of 9.68% under standard global AM 1.5 solar irradiation. Its adequate performance plus simplified synthetic procedures warrant its application in larger sized DSCs.
- This article is part of the themed collection: 2015 Journal of Materials Chemistry A Hot Papers

 Please wait while we load your content...
Please wait while we load your content...