Unusual Li-ion storage through anionic redox processes of bacteria-driven tellurium nanorods†
Abstract
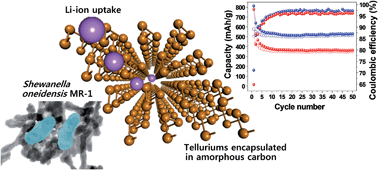
The bacterial respiration process enables the facile and morphologically-selective preparation of nanomaterials, along with the removal of environmentally toxic elements. Bacteria-driven metallic tellurium Te(0) nanorods formed extra- and intracellularly by Shewanella oneidensis MR-1, consisting of a helically-twisted atomic-wire bundle structure, exhibited distinct Li-ion uptake properties after direct or glucose-assisted surface-carbonization of bacterial cells. By synchrotron-based in situ structural characterization during cycling, it was demonstrated that the carbonized polycrystalline Te materials experience phase transition to Li2Te through simple Li-ion diffusion and charge compensation by the anionic redox reaction of metallic Te to polyanionic telluride (Ten2−). On the other hand, the carbonized amorphous Te materials show simple Li-ion accumulation around Te element with only the anionic redox reaction. The gradual generation of electrostatic interactions between Li+ and Ten2− ion pairs promotes host lattice stabilization, unlike in other metallic anode systems with volume expansion. We report that the unusual anionic redox chemistry of Te with its structural flexibility drives the reversible Li-ion uptake without any critical structural deterioration, highlighting the potential of tellurium as a new energy conversion and storage material.

 Please wait while we load your content...
Please wait while we load your content...