Enhanced methanol electrooxidation at Pt skin@PdPt nanocrystals†
Abstract
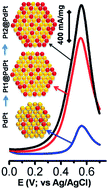
Pt skin growth over PdPt alloy nanocrystals has been described using a simple wet chemical method, where a layer-by-layer epitaxial deposition of Pt on PdPt could be understood by the Stranski–Krastanov mechanism. Initial PdPt alloy nanocrystals grown in a simple wet-chemical method, in the presence of a reducing solvent like N-methyl pyrrolidone (NMP) and a stabilizer like polyvinyl pyrrolidone (PVP), have been used as the substrate for secondary growth of a Pt thin layer. Surface changes have been observed during step-by-step growth of polyhedral Pt skin@PdPt nanocrystals originating from nearly octahedral geometries of PdPt. The methanol electrooxidation activities of two different Pt skin@PdPt nanostructures have been compared with PdPt nanocrystals with similar compositions but without skin structures and commercial RuPt catalysts. A gain factor of 8 towards electrooxidation of methanol in acidic media with activities of 1950 mA mgPt−1 and 3.1 mA cmPt−2 (with lower onset potential compared to the RuPt commercial catalyst), which is believed to be much higher compared to that of previous reports and state-of-art RuPt/C catalysts, indicating better surface properties and core-alloy formation along with improved intraparticle active interfacial sites. Additionally, exciting results of electrooxidation of ethanol and ethylene glycol with 70% and 58% activity retention respectively, after 5000 cycles are also found, demonstrating a facile C–C breaking in such C2 type alcohols.

 Please wait while we load your content...
Please wait while we load your content...