Electrolytic calcium hexaboride for high capacity anode of aqueous primary batteries†
Abstract
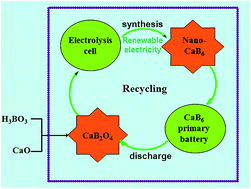
The use of lightweight elements/compounds and the employment of multi-electron reaction (MER) chemistry are two approaches used to develope high energy density materials for batteries. In this work, nanoscaled calcium hexaboride (CaB6) was prepared in one-step by the electro-reduction of solid calcium borate (CaB2O4) in molten CaCl2–NaCl. CaB2O4 was synthesized by a simple co-precipitation method. It was revealed that the electrolytic CaB6 delivers a specific capacity of 2400 mA h g−1 in 30% KOH solution though a multi-electron reaction mechanism. Its practical gravimetric energy is three times that of Zn. Decrease of particle size substantially improves both the electrochemical activity and discharge capacity of CaB6. The electrolysis of CaB2O4 in molten salt provides a straightforward and sustainable way to prepare high-capacity CaB6 using low-cost and environmentally friendly boron and calcium resources, which can be recycled from the used CaB6 primary batteries.
- This article is part of the themed collection: 2015 Journal of Materials Chemistry A Hot Papers

 Please wait while we load your content...
Please wait while we load your content...