Highly alkaline stable N1-alkyl substituted 2-methylimidazolium functionalized alkaline anion exchange membranes†
Abstract
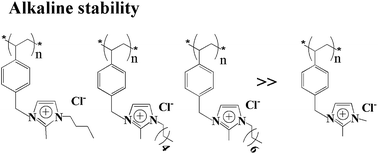
Steric hindrance and hyperconjugative effects, introduced at the N1 position of 2-methylimidazolium, greatly enhance the alkaline stability of the 2-methylimidazolium functional group. 2-Methylimidazolium small molecule compounds with N1-substituents (butyl, hexyl or octyl) are stable in 1 M KOH at 80 °C for more than 3000 h. Accordingly, the membranes based on N1-butyl, hexyl or octyl-substituted 2-methylimidzolium exhibited much more alkaline stability than membranes based on other substituted 2-methylimidazolium compounds, reflected by the almost unchanged IEC, conductivity and dimensions of the membranes after being exposed to 1 M KOH at 60 °C for hundreds of hours. This work reports the preparation of highly alkaline stable 2-methylimidazolium-based membranes by modifying the N1 position of 2-methylimidazolium.

 Please wait while we load your content...
Please wait while we load your content...