Melamine–terephthalaldehyde–lithium complex: a porous organic network based single ion electrolyte for lithium ion batteries
Abstract
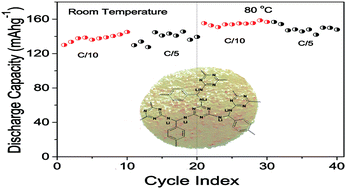
Cationic transference number and ionic conductivity of an electrolyte are among the key parameters that regulate battery performance. In the present work, we introduce a novel concept of using porous organic frameworks as a single ion-conducting electrolyte for lithium ion batteries. The synthesized lithium functionalized melamine–terephthalaldehyde framework (MTF–Li), a three dimensional porous organo–lithium complex, in a medium of organic solvent exhibits ionic conductivity comparable to the values of typical gel polymer electrolytes, and the battery cell assembled with the membrane of the material performs at both room temperature and at 80 °C. The rigid three-dimensional framework, functioning as the anionic part of the electrolyte, reduces the anionic transference number to a minimum. As a consequence, the cationic transference number increases to 0.88, close to unity. In addition, by virtue of its synthesis procedure, the electrolyte displays excellent sustainability at high temperatures, which is important for battery safety as well as for enhancing the performance and longevity of the battery.


 Please wait while we load your content...
Please wait while we load your content...