Palladium catalyst coordinated in knitting N-heterocyclic carbene porous polymers for efficient Suzuki–Miyaura coupling reactions†
Abstract
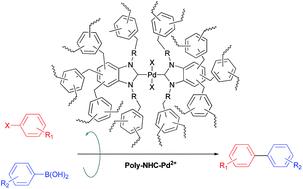
Three N-heterocyclic carbenes (NHCs) were successfully integrated into the skeleton of hyper-crosslinked polymers (HCPs) via an external cross-linking reaction. The structure and composition of the solid catalyst was characterized by SEM, N2 sorption, FT-IR, XPS and CP/MAS NMR. Depending on the nature of the NHCs, the Brunauer–Emmett–Teller (BET) surface area of HCPs could be tuned and a BET surface area as high as 1229 m2 g−1 was achieved. The Poly-NHC-2–Pd2+ catalyst afforded rapid conversion for the Suzuki–Miyaura cross-coupling reactions of various aryl halides and arylboronic acids even at a Pd loading of 0.057 mmol% in aqueous media. In particular, because of the substantial porosity and individual pore structure toward the entrapped Pd species, Poly-NHC-2–Pd2+ showed outstanding stability and recyclability, which could be reused at least 5 times without significant loss of activity. The developed microporous catalyst combined with the NHC-functionalize is one of the most efficient heterogeneous systems for Suzuki–Miyaura cross-coupling reactions of aryl halides.

 Please wait while we load your content...
Please wait while we load your content...