A comparative study of the structure, energetic performance and stability of nitro-NNO-azoxy substituted explosives†
Abstract
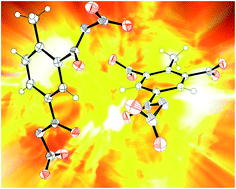
2,4-Dinitro-NNO-azoxytoluene and 2,6-dinitro-4-nitro-NNO-azoxytoluene were synthesized as energetic compounds. Their structures and properties were studied by X-ray diffractometry, nuclear magnetic resonance and infrared spectroscopy. The differences between the nitro-NNO-azoxy and nitro groups are discussed. The detonation properties, as predicted using EXPLO5, indicate that the detonation velocity and pressure of 2,4-dinitro-NNO-azoxytoluene were greater by 21.7% and 74.3%, respectively, than those of 2,4-dinitrotoluene. Nucleus independent chemical shift analysis was used to investigate skeleton aromaticity and the effect of the nitro-NNO-azoxy and nitro groups on ring aromaticity. Electrostatic potential, bond dissociation energy, Mulliken charges and Wiberg bond order were estimated by density functional theory to establish the molecular electron distribution and stabilities of the compounds. The nitro-NNO-azoxy group has a stronger electron-withdrawing property than that of the nitro group.

 Please wait while we load your content...
Please wait while we load your content...