A rechargeable Na–CO2/O2 battery enabled by stable nanoparticle hybrid electrolytes†
Abstract
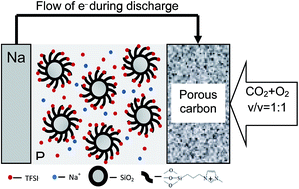
We report on rechargeable batteries that use metallic sodium as the anode, a mixture of CO2 and O2 as the active material in the cathode, and an organic–inorganic hybrid liquid as electrolyte. The batteries are attractive among energy storage technologies because they provide a mechanism for simultaneously capturing CO2 emissions while generating electrical energy. Through in and ex situ chemical analysis of the cathode we show that NaHCO3 is the principal discharge product, and that its relative instability permits cell recharging. By means of differential electrochemical mass spectrometry (DEMS) based on 12C and 13C we further show that addition of as little as 10% of 1-methyl-3-propylimidazolium bis(trifluoromethanesulfone)imide ionic liquid tethered to SiO2 nanoparticles extends the high-voltage stability of the electrolyte by at least 1 V, allowing recharge of the Na–CO2/O2 cells.

 Please wait while we load your content...
Please wait while we load your content...