Understanding the electronic and ionic conduction and lithium over-stoichiometry in LiMn2O4 spinel
Abstract
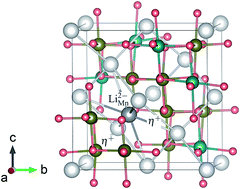
We report a first-principles study of defect thermodynamics and transport in spinel-type lithium manganese oxide LiMn2O4, an important lithium-ion battery electrode material, using density-functional theory and the Heyd–Scuseria–Ernzerhof screened hybrid functional. We find that intrinsic point defects in LiMn2O4 have low formation energies and hence can occur with high concentrations. The electronic conduction proceeds via hopping of small polarons and the ionic conduction occurs via lithium vacancy and/or interstitialcy migration mechanisms. The total conductivity is dominated by the electronic contribution. LiMn2O4 is found to be prone to lithium over-stoichiometry, i.e., lithium excess at the manganese sites, and Mn3+/Mn4+ disorder. Other defects such as manganese antisites and vacancies and lithium interstitials may also occur in LiMn2O4 samples. In light of our results, we discuss possible implications of the defects on the electrochemical properties and provide explanations for the experimental observations and guidelines for defect-controlled synthesis and defect characterization.

 Please wait while we load your content...
Please wait while we load your content...