Enthalpy of formation and thermodynamic insights into yttrium doped BaZrO3
Abstract
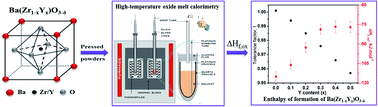
The enthalpies of formation from binary oxide components at 25 °C of Ba(Zr1−xYx)O3−δ, x = 0.1 to 0.5 solid solutions are measured by high temperature oxide melt solution calorimetry in a molten solvent, 3Na2O·4MoO3 at 702 °C. The enthalpy of formation is exothermic for all the compositions and becomes less negative when increasing yttrium content from undoped (−115.12 ± 3.69 kJ mol−1) to x = 0.5 (−77.09 ± 4.31 kJ mol−1). The endothermic contribution to the enthalpy of formation with doping content can be attributed to lattice distortions related to the large ionic radius difference of yttrium and zirconium and vacancy formation. For 0.3 ≤ x ≤ 0.5, the enthalpy of formation appears to level off, consistent with an exothermic contribution from defect clustering. Raman spectra indicate changes in short range structural features as a function of dopant content and, suggests that from x = 0.3 to 0.5 the defects begins to cluster significantly in the solid solution, which corroborates with the thermodynamic data and the drop-off in proton conductivity from x > 0.3.

 Please wait while we load your content...
Please wait while we load your content...