Sodium-ion battery cathodes Na2FeP2O7 and Na2MnP2O7: diffusion behaviour for high rate performance†
Abstract
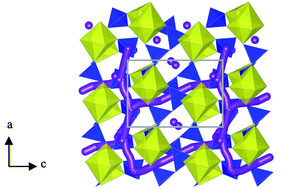
Na-ion batteries are currently the focus of significant research activity due to the relative abundance of sodium and its consequent cost advantages. Recently, the pyrophosphate family of cathodes has attracted considerable attention, particularly Li2FeP2O7 related to its high operating voltage and enhanced safety properties; in addition the sodium-based pyrophosphates Na2FeP2O7 and Na2MnP2O7 are also generating interest. Herein, we present defect chemistry and ion migration results, determined via atomistic simulation techniques, for Na2MP2O7 (where M = Fe, Mn) as well as findings for Li2FeP2O7 for direct comparison. Within the pyrophosphate framework the most favourable intrinsic defect type is found to be the antisite defect, in which alkali-cations (Na/Li) and M ions exchange positions. Low activation energies are found for long-range diffusion in all crystallographic directions in Na2MP2O7 suggesting three-dimensional (3D) Na-ion diffusion. In contrast Li2FeP2O7 supports 2D Li-ion diffusion. The 2D or 3D nature of the alkali-ion migration pathways within these pyrophosphate materials means that antisite defects are much less likely to impede their transport properties, and hence important for high rate performance.

 Please wait while we load your content...
Please wait while we load your content...