Polyhedral LiNi0.5Mn1.5O4 with excellent electrochemical properties for lithium-ion batteries†
Abstract
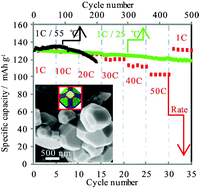
Alternative micro-sized LiNi0.5Mn1.5O4 spinels with octahedral structure (showing only one type of {111} crystal face) or chamfered polyhedral structure with extra other faces have been synthesized via a controllable method. A possible growth model, complexing–pyrolyzing-oriented, is also proposed for the formation of chamfered polyhedral LiNi0.5Mn1.5O4 spinel based on the experimental results in this paper. The chamfered polyhedral LiNi0.5Mn1.5O4 can provide a large capacity of 103 mA h g−1 even at a discharge rate as high as 50 C, which is far superior to that of the octahedral structure. Besides, the capacity retentions of the chamfered polyhedral composites are found to be 90.82% at 25 °C after 500 cycles and 90.00% at 55 °C after 200 cycles, which are also better than those of the octahedral composites. These results represent the first experimental evidence for lattice-plane anisotropy in LiNi0.5Mn1.5O4 crystals. Moreover, the pseudo-sphere structure is beneficial for obtaining high volumetric energy density and excellent processability in practical applications. In short, we have revealed for the first time that, through chamfering an octahedron to a pseudo-sphere-like polyhedron rather than doping or coating, a micro-sized LiNi0.5Mn1.5O4 spinel with good compatibility between energy/power density and cycle life can be synthesized successfully without sacrificing other properties.

 Please wait while we load your content...
Please wait while we load your content...