Tunable electronic and magnetic properties of graphene-like ZnO monolayer upon doping and CO adsorption: a first-principles study
Abstract
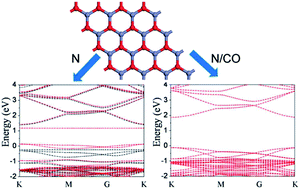
Graphene-like zinc oxide monolayer (g-ZnO) is a new class of two-dimensional nanomaterials with unique new properties that is still largely unknown. This work studies the tunability of the electronic and magnetic properties of g-ZnO upon chemical doping (with B, N and C) and CO adsorption by using first-principles calculations. Both electronic and magnetic properties of g-ZnO exhibit strong dependency on its structural change and molecular adsorption. The g-ZnO with oxygen atom substituted by a C or N atom (one atom per supercell) are ferromagnetic (FM) half metal (HM), while that substituted by a B atom is an FM semiconductor. The doped g-ZnO shows strong chemisorption to CO molecule by forming A–CO bond (A = B, N or C), in contrast to the weak physisorption of the intrinsic g-ZnO. Furthermore, CO adsorption converts the N- and C-doped g-ZnO to n-type semiconductor with nonmagnetic (NM) ground states, while B-doped g-ZnO becomes a ferromagnetic half metal (FM-HM). The mechanism for property change has been investigated by analyzing their partial density of states (PDOS) upon different conditions. This study provides insights in the physical properties and chemical reactivity of g-ZnO, which could help in realizing their diverse potentials in electronic and magnetic devices.

 Please wait while we load your content...
Please wait while we load your content...