First principles study of dopant solubility and defect chemistry in LiCoO2
Abstract
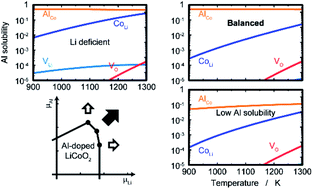
Doping and alloying are extensively applied to electrode active materials to improve the performance of lithium-ion batteries. Thus, the defect formation energy and equilibrium concentrations of doped ions in LiCoO2 are estimated as extrinsic point defects, as well as those of the native defects, using first-principles calculations. Na, K, Rb, Mg, Ca, Sr, Zn, Al, Ga, In, Sc, Y, Zr and Nb are selected as the dopants with a variety of valences and ionic radii. Dopants with a solubility higher than 1% are Al and Ga for the Co site, and Na and Zn for the Li site at 1100 K and 0.2 atm oxygen pressure, without a significant increase in lithium deficiency. Mg shows a solubility of about 0.5% at both sites, and the preferential occupation site depends on conditions: the Co site under oxidative conditions, whereas the Li site is preferred under reductive conditions. The estimated dopant solubility indicates that the valences of the dopants are the predominant factor for the occupation sites, whereas the ionic radii have a significant effect on the solubility. The dopant solubility and defect concentrations strongly depend on the chemical potential. Therefore, an appropriate ratio of starting materials that provides the desirable chemical potentials is necessary to achieve the balance of high dopant solubility and low lithium deficiency. The present calculation, based on the first-principles calculations, can provide quantitative information about the balance of the defects and also about the proper synthetic and post-synthetic conditions.

 Please wait while we load your content...
Please wait while we load your content...