Carbon dioxide mediated, reversible chemical hydrogen storage using a Pd nanocatalyst supported on mesoporous graphitic carbon nitride†
Abstract
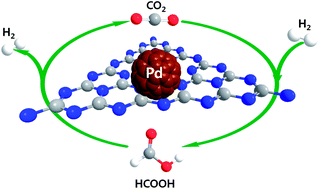
Reversible, carbon dioxide mediated chemical hydrogen storage was first demonstrated using a heterogeneous Pd catalyst supported on mesoporous graphitic carbon nitride (Pd/mpg-C3N4). The Pd nanoparticles were found to be uniformly dispersed onto mpg-C3N4 with an average size of 1.7 nm without any agglomeration and further exhibit superior activity for the dehydrogenation of formic acid with a turnover frequency of 144 h−1 even in the absence of external bases at room temperature. Initial DFT studies suggest that basic sites located at the mpg-C3N4 support play synergetic roles in stabilizing reduced Pd nanoparticles without any surfactant as well as in initiating H2-release by deprotonation of formic acid, and these potential interactions were further confirmed by X-ray absorption near edge structure (XANES). Along with dehydrogenation, Pd/mpg-C3N4 also proves to catalyze the regeneration of formic acid via CO2 hydrogenation. The governing factors of CO2 hydrogenation are further elucidated to increase the quantity of the desired formic acid with high selectivity.

 Please wait while we load your content...
Please wait while we load your content...