Amorphous and highly nonstoichiometric titania (TiOx) thin films close to metal-like conductivity
Abstract
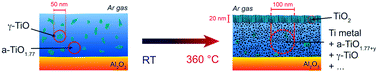
Oxygen-deficient titanium oxide films (TiOx) have been prepared by pulsed laser deposition at room temperature. Samples in their as-deposited state have an average composition of TiO1.6, are optically absorbing and show electronic conductivities in the range of 10 S cm−1. The films are metastable and consist of grains of cubic titanium monoxide (γ-TiO) embedded in an amorphous TiO1.77 matrix. Upon annealing in an argon atmosphere the electrical conductivity of the films increases and comes close to metal-like conductivity (1000 S cm−1) at about 450 °C whereas the local structure is changed: nanocrystalline grains of metallic Ti are formed in the amorphous matrix due to an internal solid state disproportionation. The highly conductive state can be frozen by quenching. During heat treatment in an argon atmosphere a stoichiometric rutile TiO2 surface layer forms due to oxidation by residual oxygen. The combination of a highly conductive TiOx film with such an approximately 20 nm thick rutile cover layer leads to a surprisingly high efficiency for the water-splitting reaction without the application of an external potential.

 Please wait while we load your content...
Please wait while we load your content...