Enhanced activity and stability of Pt–La and Pt–Ce alloys for oxygen electroreduction: the elucidation of the active surface phase†
Abstract
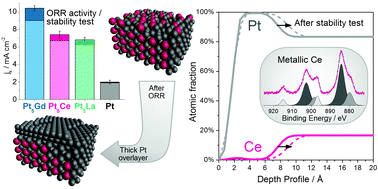
Three different Pt–lanthanide metal alloys (Pt5La, Pt5Ce and Pt3La) have been studied as oxygen reduction reaction (ORR) electrocatalysts. Sputter-cleaned polycrystalline Pt5La and Pt5Ce exhibit more than a 3-fold activity enhancement compared to polycrystalline Pt at 0.9 V, while Pt3La heavily corrodes in 0.1 M HClO4 electrolyte. Angle Resolved X-ray Photoelectron Spectroscopy (AR-XPS) and Low Energy Ion Scattering (LEIS) have been extensively combined with electrochemical techniques to follow the chemical and structural changes at the surface. The highly reactive lanthanide atoms are not stable in the presence of oxygen and readily oxidize. The surface oxides are completely dissolved in the electrolyte. In Pt5La and Pt5Ce the so formed Pt overlayer provides kinetic stability against the further oxidation and dissolution. At the same time, it ensures a very high stability during ORR potential cycling, suggesting that these alloys hold promise as cathode catalysts in Proton Exchange Membrane Fuel Cells (PEMFCs).
- This article is part of the themed collection: 2021 Journal of Materials Chemistry Lectureship winner: María Escudero Escribano

 Please wait while we load your content...
Please wait while we load your content...