Synthesis and electrocatalytic performance of MnO2-promoted Ag@Pt/MWCNT electrocatalysts for oxygen reduction reaction
Abstract
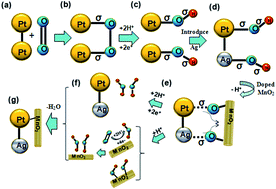
Two forms of crystalline MnO2 were synthesized by a hydrothermal method, and MnO2-doped Ag@Pt/MWCNT composites were prepared by ultrasonic treatment for oxygen reduction reaction in proton exchange membrane fuel cells (PEMFC). The morphology of the electrocatalyst samples was characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and high resolution transmission electron microscopy (HRTEM). We found that the Ag@Pt/MWCNTs catalyst exhibited a core–shell nanostructure and the two forms of MnO2 were α-MnO2 and β-MnO2. The electrochemical properties of the electrocatalyst samples were studied by cyclic voltammetry (CV), linear sweep voltammetry (LSV), chronoamperometry (CA), and electrochemical impedance spectroscopy (EIS) in an acidic medium. The results demonstrated that Ag10%@Pt10%/MWCNTs-α-MnO220% had the largest electrochemical activity area (85.83 m2 g−1) of all electrocatalysts and the oxygen reduction reaction on the electrocatalyst proceeds through a 4e− reduction pathway.

 Please wait while we load your content...
Please wait while we load your content...