Facile preparation of PdNi/rGO and its electrocatalytic performance towards formic acid oxidation†
Abstract
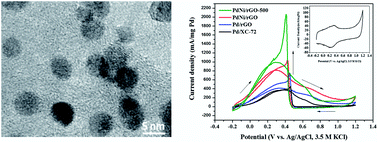
A highly dispersed and active PdNi alloy supported on reduced graphene oxide (rGO) was successfully prepared for formic acid electro-oxidation under acidic conditions. The material was prepared by a simultaneous reduction method using NaBH4 as the reductant, followed by annealing in H2 at 500 °C. Different characterization methods verified that H2 annealing at an appropriate temperature had a significant influence on both physiochemical composition and electrocatalytic performance of the synthesized catalysts. H2 annealing at 500 °C facilitated formation of a PdNi alloy without agglomeration of the nano-sized metallic catalyst, while without H2 treatment Ni was found predominantly in the form of Ni(OH)2. Cyclic voltammetry and chromoamperometric testing demonstrated that, when compared to the sample without H2 treatment, PdNi/rGO after H2 annealing exhibited better catalytic activity and stability towards formic acid electro-oxidation. The enhanced performance can be explained by the modification of the electronic structure of Pd by Ni after alloy formation and the large specific surface area and excellent electron conductivity of the rGO support.

 Please wait while we load your content...
Please wait while we load your content...