Stable blue TiO2−x nanoparticles for efficient visible light photocatalysts†
Abstract
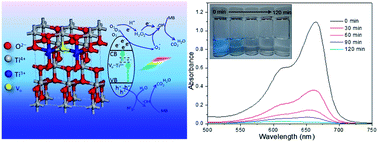
Tailored fabrication of non-stoichiometric semiconductors has attracted considerable interest since the oxygen vacancy is the fundamental and intrinsic defect in reduced semiconductors and has critical impacts on their physicochemical properties such as tuning optical absorption, increasing conductivity, etc. Therefore, it is highly important to have a comprehensive understanding of the methods and the techniques of Ti3+ generation as well as Ti3+ property exploration. Here we have developed an effective strategy for the large-scale synthesis of blue titania with Ti3+ localized in the core of TiO2−x nanoparticles (NPs) based on Le Chatelier's principle. Electron paramagnetic resonance (EPR) spectra confirm the presence of Ti3+ in as-prepared samples, which is attributed to be the origin of its excellent visible-light photocatalytic activity. Further analysis based on X-ray photoelectron spectroscopy (XPS) spectra of Ti 3d indicates that only the rhombic Ti3+ is localized in the bulk, rather than on the surface of obtained TiO2−x NPs. It can be inferred that the electronic structure of blue titania NPs is determined by the unique defective and non-stoichiometric TiO2−x core and stoichiometric TiO2 shell structure. As a consequence, our samples show excellent stability and retain their blue color upon storage in ambient atmosphere for at least one year. The structure, crystallinity and morphology of the as-prepared samples were characterized systematically. The blue TiO2−x NPs exhibit higher photocatalytic activity for the photooxidation of methylene blue than the commercial P25 NPs under visible light irradiation (λ ≥ 400 nm). It is found that the molar ratio of Ti4+–Ti3+ in the reaction system plays a key role in the photocatalytic activity of Ti3+ self-doped TiO2−x under visible light since the electronic structures of the resulting TiO2−x NPs can be finely tuned by the molar ratios of Ti4+–Ti3+ in the precursors. The optimal molar ratio of Ti4+–Ti3+ is 1 : 40, at which the obtained titania NPs display a deep blue appearance and the highest catalytic activity. Therefore, our present work highlights the feasibility of simultaneous engineering of surface energetics and optical properties for designing novel TiO2-based nanomaterials.

 Please wait while we load your content...
Please wait while we load your content...