Self-assembled graphene and LiFePO4 composites with superior high rate capability for lithium ion batteries†
Abstract
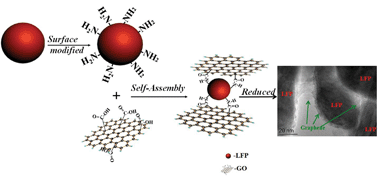
A graphene encapsulated LiFePO4 composite has been synthesized by self-assembly of surface modified LiFePO4 and graphene oxide with peptide bonds, followed by reduction. The graphene forms a continuous conductive coating network connecting the LiFePO4 nanoparticles to facilitate electron transportation, resulting in excellent high rate capability with 70% capacity retention at 50 C rate. The apparent activation energy of the graphene encapsulated LiFePO4 composite (9.6 kJ mol−1) is much lower than that of the carbon coated LiFePO4 (14.6 kJ mol−1). An excellent cycling performance is also demonstrated, in which the capacity loss is less than 8.6% after 950 cycles at 10 C. Therefore, this hybrid material is promising for use as a cathode material for high rate lithium ion batteries.

 Please wait while we load your content...
Please wait while we load your content...