Mesoporous Mn3O4–CoO core–shell spheres wrapped by carbon nanotubes: a high performance catalyst for the oxygen reduction reaction and CO oxidation†
Abstract
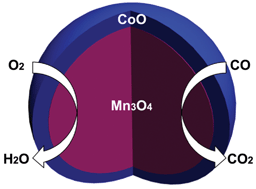
The controllable synthesis of transition metal oxide nanomaterials has attracted considerable attention for the replacement of the current precious metal catalysts. Herein, we have developed a facile method to successfully synthesize Mn3O4–CoO core–shell mesoporous spheres, which are wrapped by carbon nanotubes (CNT), and investigated the catalytic activity for the oxygen reduction reaction (ORR) and CO oxidation for the first time. The ORR process on the Mn3O4–CoO/CNT catalysts was via a complete oxygen reduction process (4e−), and the catalytic activity was far better than for the Mn3O4/CNT and CoO/CNT catalysts. The durability even out-performed the commercial Pt/C catalysts. As compared with the Mn3O4/CNT and CoO/CNT catalysts, the Mn3O4–CoO/CNT catalysts also exhibited better catalytic activity for CO oxidation. The initial and complete conversion temperatures for the Mn3O4–CoO/CNT catalysts can decrease to 30 and 120 °C, respectively. The good catalytic activity for the ORR and CO oxidation is due to the high specific surface area (138.9 m2 g−1) provided which gives many catalytically active sites, mesoporous structure (15 to 120 nm) favoured for molecule accessibility to the active surface of the nanocrystals and mass transport, and the synergistic catalytic effect of Mn3O4 and CoO catalytically active sites.

 Please wait while we load your content...
Please wait while we load your content...