Fabrication of BixPtyPdz alloy nanoporous plates with electro-catalytic activity†
Abstract
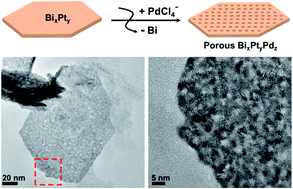
We demonstrate the fabrication of Bi21Pt35Pd44 alloy porous nanoplates by a galvanic replacement method using Bi65Pt35 bimetallic nanoplates as the sacrificial template. Bi65Pt35 nanoplates, prepared by reduction with diol (1,2-hexadecanediol) at low temperature (100 °C), were converted to the porous nanoplate structure by a K2PdCl4/water/ethanol (1/1, v/v) mixed solution via galvanic replacement between bismuth and palladium. The formation of Bi21Pt35Pd44 porous nanoplates is attributed to disperse crystallization under conditions of high nucleation rate. The electro-chemically active surface area (ECSA) for the Bi21Pt35Pd44 alloy porous nanoplate is measured to be 0.943 cm2. This value is 3 times greater than the ECSA (0.283 cm2) of the commercial Pt/C (Alpha, Pt 20%) electrode. The electro-catalytic activity of the prepared porous nanoplates was examined for the oxidation of methanol in acidic solution. The Bi21Pt35Pd44 porous nanoplates can effectively catalyze oxidation of methanol in acidic solution, probably owing to their larger surface area and many active reaction sites. The Bi21Pt35Pd44 porous nanoplates provide a new form of unsupported catalysts in contrast to the traditional supported nanoparticle catalyst and can be uniformly synthesized and separated easily from the reaction mixtures without aggregation.

 Please wait while we load your content...
Please wait while we load your content...