An extremely stable MnO2 anode incorporated with 3D porous graphene-like networks for lithium-ion batteries†
Abstract
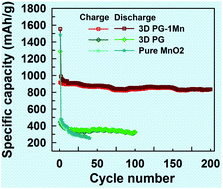
A rational design of MnO2/3D porous graphene-like (PG) (denoted as 3D PG–Mn) composites and their fabrication via a simple and cost-effective redox process have been achieved for the first time. The 3D PG can provide a highly conductive structure in conjunction with a large surface area to support good contact between the MnO2 nanoparticles and effectively enhance the mechanical strength of the composite during volume changes as well as suppress the aggregation of MnO2 nanoparticles during Li ion insertion/extraction. As a result, the 3D PG–Mn composite with a content of 62.7 wt% MnO2 shows a highly stable capacity of up to 836 mA h g−1 after 200 cycles at a current density of 100 mA g−1 and reversible high rate charge–discharge performance. Such a highly stable 3D PG–Mn composite can be produced on a large-scale and might have even wider applications as an anode material in lithium-ion batteries.

 Please wait while we load your content...
Please wait while we load your content...