Highly active reduction of oxygen on a FeCo alloy catalyst encapsulated in pod-like carbon nanotubes with fewer walls†
Abstract
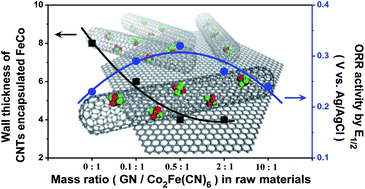
Employing an alternative of the Pt-based electrocatalysts for oxygen reduction reaction (ORR) has become a major interest in the fundamental research of the polymer electrolyte membrane fuel cells (PEMFCs). The carbon-encapsulated metal catalyst, on which O2 is readily activated by the electrons transferred from the metal to the carbon surface, has recently been demonstrated as a promising strategy to produce robust non-precious metal electrocatalysts. However, the thickness of carbon walls might affect the process of the electron transfer, and subsequently the ORR activity. It is thus vital to explore the influence of the carbon wall thickness on the ORR reactivity for further improvement in designing carbon-encapsulated non-precious metal catalysts for ORR. Herein, we report a novel FeCo alloy catalyst encapsulated in pod-like carbon nanotubes via introducing graphene nanosheets into the raw materials to tailor the carbon wall thickness. The ORR activity of these catalysts increases drastically with the decreased thickness of the carbon walls, which could be attributed to the enhanced adsorption of O2 on the carbon surface upon decreasing the carbon wall thickness. These findings provide a route for the rational design of high-performance non-precious metal cathode catalysts in PEMFCs.

 Please wait while we load your content...
Please wait while we load your content...