Evidence for oxygen reduction reaction activity of a Ni(OH)2/graphene oxide catalyst†
Abstract
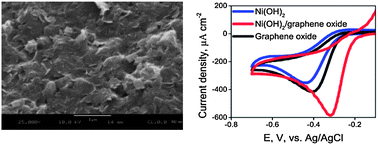
Oxygen reduction reaction catalysis on a microwave synthesized Ni(OH)2/graphene oxide material was investigated via cyclic voltammetry, rotating disk electrode measurements, chronoamperometry, and electrochemical impedance spectroscopy. Cyclic voltammetry in an 0.5 M alkaline solution indicated that the Ni(OH)2/graphene oxide material possesses significant oxygen reduction reaction activity as evidenced by a peak potential of −310 mV vs. Ag/AgCl. This value was a shift of +110 mV as compared to the unsupported Ni(OH)2 nanoparticles and +90 mV as compared to the graphene oxide support alone. Rotating disk electrode studies show that the limiting current density of the Ni(OH)2/GO catalyst is 1.3 mA cm−2 and the electron transfer number is 3.5. Chronoamperometry demonstrates that the current density attributable to the oxygen reduction reaction on the Ni(OH)2/graphene oxide material sustained a steady state value of 60% of its initial value. Electrochemical Impedance spectroscopy showed that the charge transfer resistance of the Ni(OH)2/graphene oxide catalyst was significantly lower than either the Ni(OH)2 nanoparticles or the graphene oxide support. The electrocatalytic properties of the Ni(OH)2/graphene oxide material are discussed in the context of specific chemical interaction between the Ni(OH)2 nanoparticles and the graphene oxide support.

 Please wait while we load your content...
Please wait while we load your content...