A versatile protocol for the ionothermal synthesis of nanostructured nickel compounds as energy storage materials from a choline chloride-based ionic liquid†
Abstract
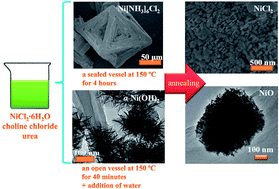
A versatile ionothermal strategy is proposed to synthesize nanostructured nickel compounds as energy storage materials, such as Ni(NH3)6Cl2 crystals, nanosheet-like NiCl2, nanoflower-like (NF) α-Ni(OH)2, and mesoporous NF-NiO. The primal solution is a choline chloride (ChCl)–urea mixture-based ionic liquid with nickel chloride hexahydrate (NiCl2·6H2O) dissolved. The proposed ionothermal protocol is attractive and environmental friendly because ChCl and urea are naturally biocompatible compounds. It has been reported that Ni(NH3)6Cl2 is an indirect and reversible hydrogen storage material for fuel cells. Herein, we demonstrate that Ni(NH3)6Cl2 crystals with octahedral geometry and an open structure can be obtained by maintaining the primal solution in a sealed vessel at 150 °C for 4 hours. A kinetic-to-dynamic growth mechanism is proposed to elucidate the formation of the interesting Ni(NH3)6Cl2 structure. Further annealing of the Ni(NH3)6Cl2 crystals at 300 °C produces sheet-like NiCl2, which can be utilized as an active material in sodium-ion batteries. Interestingly, with the addition of a small amount of water into the primal solution which has been kept at 150 °C and open to the air for 40 minutes, NF α-Ni(OH)2 can be obtained. Further annealing of the NF α-Ni(OH)2 at 300 °C leads to the formation of NF-NiO with a mesoporous structure, which is demonstrated to be a promising pseudo-capacitive electrode material in a KOH aqueous solution. The rapid activation process, good cycling performance, and high specific capacitance of the NF-NiO electrode are attributed to its high surface area (72.9 m2 g−1) and the mesoporous structure. We expect that the ionothermal method will have implications for the use of ChCl–urea mixture-based ionic liquids in the large-scale synthesis of advanced functional materials.

 Please wait while we load your content...
Please wait while we load your content...