Li2MnSiO4@C nanocomposite as a high-capacity cathode material for Li-ion batteries†
Abstract
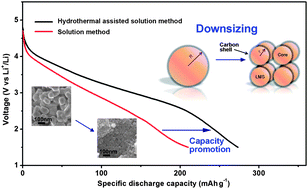
In this paper, we report on the preparation of a Li2MnSiO4@C nanocomposite and its application as a high-capacity cathode material for rechargeable Li-ion batteries. Li2MnSiO4@C was synthesized via a hydrothermal-assisted solution route by using ascorbic acid as a carbon source and reductant. Based on the characterization by XRD, SEM, TEM, BET, elemental analysis and Raman analysis, it was found that the as-obtained homogenous Li2MnSiO4@C nanocomposite was composed of a Li2MnSiO4 core in a high-purity phase and a graphitized carbon shell. The electrochemical measurement results showed that Li2MnSiO4@C with an average particle size of 22.8 nm exhibited enhanced electrochemical properties, with initial discharge capacities of 281.5 mA h g−1 at 25 °C and 321.4 mA h g−1 at 45 °C, suggesting two lithium ions per formula unit.

 Please wait while we load your content...
Please wait while we load your content...