On the H2/D2 isotopic exchange rate of proton conducting barium cerates and zirconates
Abstract
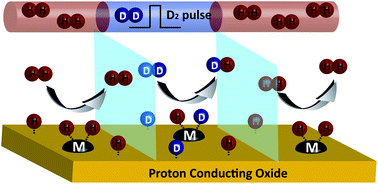
H2/D2 pulse isotope exchange experiments were employed for the first time to gain insight into the hydrogen oxidation mechanism of proton conducting solid oxide fuel cell electrode materials. Phase pure perovskites, BaCeO3 (BC), BaCe0.9Y0.1O3−δ (BCY), BaZrO3 (BZ) and BaZr0.9Y0.1O3−δ (BZY) were synthesized. The experiment was performed in both dry and wet H2, isotopic exchange was clearly observed over all samples between 250 and 525 °C in dry H2, 425 and 725 °C in wet H2. The conversion of D2 reached above 88% at 375 °C in BC and BCY, 550 and 425 °C in BZ and BZY respectively; however, no significant difference in rate was observed between the materials. Surface composition analysis via High Sensitivity-Low Energy Ion Scattering (HS-LEIS) and X-ray photoelectron spectroscopy (XPS) revealed significant Ba enrichment on the outermost surface (71.1–87.1%, fractional surface coverage of Ba). It is suggested that this similar surface composition leads to similar catalytic activity. Significant rate enhancement was observed upon 1 wt% surface doping with active transition metal catalysts, Ni, Pt and Co. We suggest that hydrogen dissociation occurs primarily on the metallic component of H+-SOFC cermet anodes.

 Please wait while we load your content...
Please wait while we load your content...