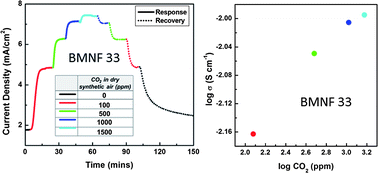
Mixed ionic-electronic conducting BaMg0.33Nb0.67−xFexO3−δ was successfully synthesized by conventional solid state method in air at elevated temperature. Fe-doping helped to increase the total conductivity, while the chemical stability under CO2 at elevated temperatures and under H2O vapour at boiling conditions decreased with increasing Fe content above x = 0.17 in BaMg0.33Nb0.67−xFexO3−δ. Maximum total conductivity of 10−3 S cm−1 at 300 °C was obtained for BMNF33 in air, and further increase in Fe content lowered the electrical conductivity, particularly at low temperatures. Activation energy, in the range of 300–700 °C, for total electrical conduction was found to decrease in air by the incorporation of Fe in BaMg0.33Nb0.67O3 (BMN) (BMN: 0.96 eV; BMNF17: 0.72 eV; BMNF33: 0.25 eV). Furthermore, the x = 0.17 composition demonstrated excellent CO2 sensing characteristics at 700 °C. The investigated perovskite-type metal oxides seem to be promising candidates for monitoring CO2 (ppm level in air) at elevated temperatures.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...