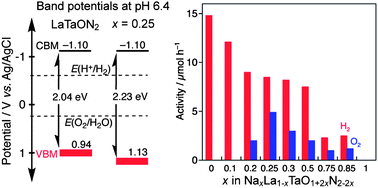
A series of oxynitride solid solutions, NaxLa1−xTaO1+2xN2−2x with x = 0.1, 0.2, 0.25, 0.3, 0.5, 0.75 and 0.85, was successfully synthesized by nitridation of amorphous oxide precursors under an NH3 stream at 1273 K. The band gaps of the solid solutions increased with the Na content. Photoelectrochemical analysis was conducted to obtain information on the band potentials of the solid solutions. The valence band maxima of the solid solutions were at more positive potentials than that of LaTaON2 (x = 0). Solid solutions of NaxLa1−xTaO1+2xN2−2x with x = 0.20–0.85 exhibited photocatalytic activity for both H2 and O2 evolution under visible light irradiation in the presence of sacrificial reagents, while LaTaON2 had no activity for O2 evolution. The achievement of O2 evolution with these solid solutions is due to control of the valence band potential; therefore, control of the photocatalytic properties of LaTaON2 was achieved by the formation of solid solutions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...