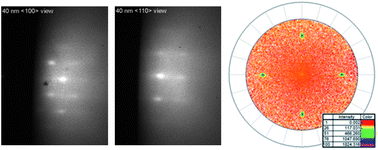
In this paper, the formation of YBiO3 starting from a new water-based precursor was studied in both bulk and thin films under different processing conditions. The decomposition of this precursor was studied with thermogravimetric and differential thermal analysis and the reaction products were identified with X-ray diffraction. Our results show that starting from our water-based solutions, polycrystalline YBO powders can be obtained in air and argon atmospheres. Once the partial oxygen pressure was reduced using an Ar–5% H2 mixture, reduction of Bi3+ was observed, restricting YBO formation. When thin film synthesis on single crystal LaAlO3 is performed, clear differences in processing conditions were observed regarding powder synthesis. The addition of water vapour to the argon gas is necessary in order to suppress Bi2O3 sublimation. Using the appropriate synthesis conditions, well-textured, homogeneous, dense and smooth (Ra = 2.6–1.8 nm) films of 140 nm and 40 nm thickness are obtained at sinter-temperatures as low as 750 °C. YBa2Cu3O7−x deposited by Pulsed Laser Deposition on the 40 nm thick YBiO3 films yielded a Jc (77 K) of 3.6 MA cm−2 in self-field, indicating the potential of this material as an alternative for CeO2 in the CeO2/La2Zr2O7/Ni–5%W and CeO2/YSZ/Stainless Steel coated conductor architectures.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...