Unraveling structural evolution of LiNi0.5Mn1.5O4 by in situneutron diffraction†
Abstract
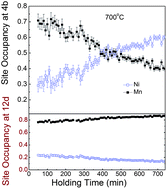
The electrochemical properties of the spinel LiNi0.5Mn1.5O4 cathode material are influenced by the synthesis processes, which determines the impurity phase and the distribution of Ni and Mn in the spinel structure. Taking advantage of neutron's high sensitivity to Ni and Mn, in situ

 Please wait while we load your content...
Please wait while we load your content...