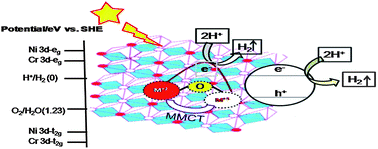
In this work we have prepared a series of visible-light active Ni–Zn/Cr–CO32− LDHs with (Ni + Zn)/Cr ratio 2.0, while varying the Ni/Zn atomic ratio to 0 : 100, 25 : 75, 50 : 50, 75 : 25 and 100 : 0, and tested them for visible light photocatalytic hydrogen evolution. The photophysical and photocatalytic properties of the samples were evaluated thoroughly by PXRD, TEM and FESEM, UV-vis DRS, FTIR, PL and BET-Surface area. The PXRD measurement demonstrates a characteristic of hydrotalcite with long range order structure. The shifting of the d110 plane towards a lower angle clearly indicates that there is Ni2+ substitution in the brucite layer of Zn/Cr–CO32− LDH. Different diffuse reflectance spectra revealed that the absorption in the visible region is attributed to the metal-to-metal charge-transfer (MMCT) excitation of ZnII/NiII–O–CrIII to ZnI/NiI–O–CrIV. This oxo-bridged hetero-bimetallic assembly acts as a photo-induced centre for hydrogen evolution. The photocatalytic studies suggested that the Ni–Zn/Cr–CO32− LDH with Ni : Zn ratio of 75 : 25 exhibits the best photocatalytic activity under visible light radiation compared with the others. The detailed mechanism for the photocatalytic decomposition of water to hydrogen has also been discussed.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...