Mixed-metal (Li, Al) amidoborane: synthesis and enhanced hydrogen storage properties†
Abstract
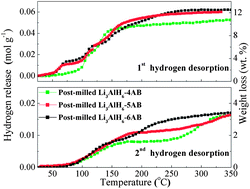
Mixed-metal (Li, Al) amidoborane has been synthesized via mechanical ball milling of ammonia borane with lithium hexahydridoaluminate in different molar ratios. The reversible dehydrogenation properties of the thus-synthesized metallic amidoborane and its mixtures with ammonia borane in different ratios were systematically investigated in comparison with neat ammonia borane (AB). On the basis of thermogravimetric analysis and mass spectrometry results, the thus-synthesized mixed-metal amidoborane was shown to release around 10 wt% hydrogen below 200 °C, with an effective suppression of volatile side products. Furthermore, a synergistic effect between metallic amidoborane and ammonia borane has been identified, which leads to the release of 9 wt% hydrogen with high purity at 120 °C. Additionally, upon treatment with hydrazine in liquid ammonia, the regenerated products from the decomposed Li3AlH6–nAB (n = 4, 5, and 6) composites can release 3.5 wt% hydrogen with high purity, corresponding to an approximate 35%, 30%, and 26% regeneration yield for the post-milled Li3AlH6–nAB (n = 4, 5, and 6) composites, respectively.

 Please wait while we load your content...
Please wait while we load your content...