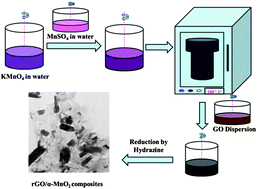
The electrochemical performance of rechargeable Li–air batteries containing a reduced graphene oxide (rGO)/α-MnO2 composite and neat α-MnO2 electrode is studied. The rGO/α-MnO2 composite exhibits a specific capacity as high as 558.4 mA h g−1 at a current density of 100 mA g−1, indicating its potential to make a good cathode material. The composite electrode also presents a relatively moderate degradation of capacities with increasing cycles, compared to the neat α-MnO2 electrode. rGO functions as the conducting medium to connect the α-MnO2 nanorods, thus improving the Li ion transfer. The mechanisms responsible for the capacity degradation in the composite electrodes are studied after a series of interrupted charge/discharge cycles, detecting Li2O2 and LiF as the main reaction products formed on the electrode surface. In particular, the LiF layer is identified to be an important component of reaction products, which serves as a barrier to reactions between the Li ions and electrons with the electrode, giving rise to detrimental effects on the cyclic and capacity performance of Li–air batteries.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...