oCVD poly(3,4-ethylenedioxythiophene) conductivity and lifetime enhancement via acid rinse dopant exchange†
Abstract
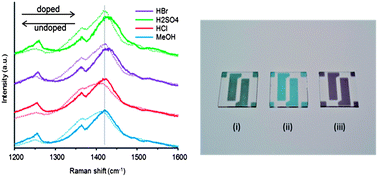
Reduced sheet resistance and longer film stability of oCVD (oxidative chemical vapour deposition) PEDOT films were achieved by including a post-process acid rinse step in the production of the thin films. PEDOT films were rinsed in multiple concentrations of hydrobromic acid, sulfuric acid, and hydrochloric acid to test the effect of acid rinsing on sheet resistance, doping concentration, chemical composition, optical transmittance, and film morphology. XPS, FTIR, Raman spectroscopy, and XRD measurements were taken to determine the morphology and composition of the rinsed films. On average, rinsing films in HCl, HBr, and H2SO4 produced conductivity increases of 37%, 135%, and 117%. The dc to optical conductivity ratio, σdc/σop, was increased to 6, 12, and 10, for HCl, HBr, and H2SO4 rinsed films respectively as compared to σdc/σop = 4 for MeOH rinsed films. This study found evidence of dopant exchange within the films facilitated by the acid rinsing step, as well as complete removal of residual iron chloride oxidant. The acid rinse step also resulted in improved film conductivity stability at elevated temperatures. The XRD measurements in particular show signs of semi crystallinity in the PEDOT film after acid rinsing in comparison to an amorphous structure observed before this step. In this study, acid rinsing applied as a post-process step alters thin PEDOT films in ways that enhance their ability to function as electrode materials in photovoltaic devices.

 Please wait while we load your content...
Please wait while we load your content...