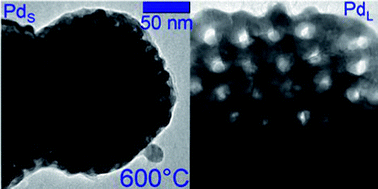
Palladium and its alloys have high-value applications as materials for high-performance hydrogen storage, chromatographic separation of hydrogen isotopes, electrocatalysis and catalysis. These materials can be formed by chemical or electrochemical reduction in a lyotropic liquid crystalline template that constrains their growth on the nanometer scale. This approach works for a variety of metals, but Pd presents special challenges due to the autocatalytic nature of its growth, which can disrupt the template structure, resulting in disordered pores. Presented herein is a scaleable synthesis that overcomes these challenges, yielding mesoporous Pd powder having pore diameters of 7 or 13 nm. Pore size control is effected by varying the size of the molecular template, polystyrene-block-polyethylene oxide. We have used heated-stage TEM for in situ observation of the materials in vacuum and in the presence of H2 gas, demonstrating that both pore diameter and the chemical state of the surface play important roles in determining thermal stability. Improved stability compared to previously reported examples facilitates preparation of scalable quantities of regularly mesoporous Pd that retains porosity at the elevated temperatures required for applications in hydrogen charge/discharge and catalysis.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...