Polyphilic hydrogen bonded block molecules involving semiperfluorinated and silylated molecular fragments†
Abstract
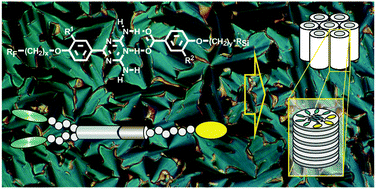
2,4-Diamino-6-phenyl-1,3,5-triazines grafted with one or two semiperfluorinated chains at the phenyl substituent have been investigated in binary mixtures with one-chain and two-chain oligo(dimethylsiloxy) terminated 4-alkoxy- and 3,4-dialkoxybenzoic acids. Equimolar mixtures of the triazines with the complementary acids form discrete double hydrogen-bonded heterodimers with an elongated central core. Mesomorphic properties are observed only if at least two terminal fluoroalkyl chains are attached to the triazine entity. Three to four heterodimers generate aggregates with preferably parallel adjusted rod-like H-bonded cores. The space requirement of the segregated peripheral fluoroalkyl and oligosiloxane segments considerably exceeds that of the central core region, leading to an overall circular geometry of the associates which organize in columnar LC phases. The chains initiate a local segregation which is however not long range and retains a time and space averaged two-dimensional hexagonal lattice.


 Please wait while we load your content...
Please wait while we load your content...