Highly stretchable hydrogels from complex coacervation of natural polyelectrolytes†
Abstract
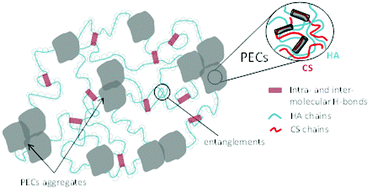
The controlled complex coacervation of oppositely charged hyaluronic acid (Mw ≈ 800–1000 kg mol−1) and chitosan (Mw ≈ 160 kg mol−1, degree of acetylation = 15%) led to hydrogels with controllable properties in terms of elasticity and strength. In this work, we performed desalting by dialysis of high ionic strength solutions of mixed polyelectrolytes and showed that the control of the pH during the polyelectrolyte assembly greatly impacts the mechanical properties of the hydrogel. First, for pHs from 5.5 to 7.5, a slight coacervation was observed due to low chitosan protonation and poor polyelectrolyte associations. Then, for pHs from 3.0 to 5.5, coacervation and syneresis led to free-standing and easy to handle hydrogels. Finally, for pHs from 2.0 to 3.0 (close to the pKa of the hyaluronic acid), we observed the unusual stretchability of these hydrogels that could arise from the pre-folding of hyaluronic acid chains while physical crosslinking was achieved by hyaluronic acid/chitosan polyelectrolyte complexation.


 Please wait while we load your content...
Please wait while we load your content...